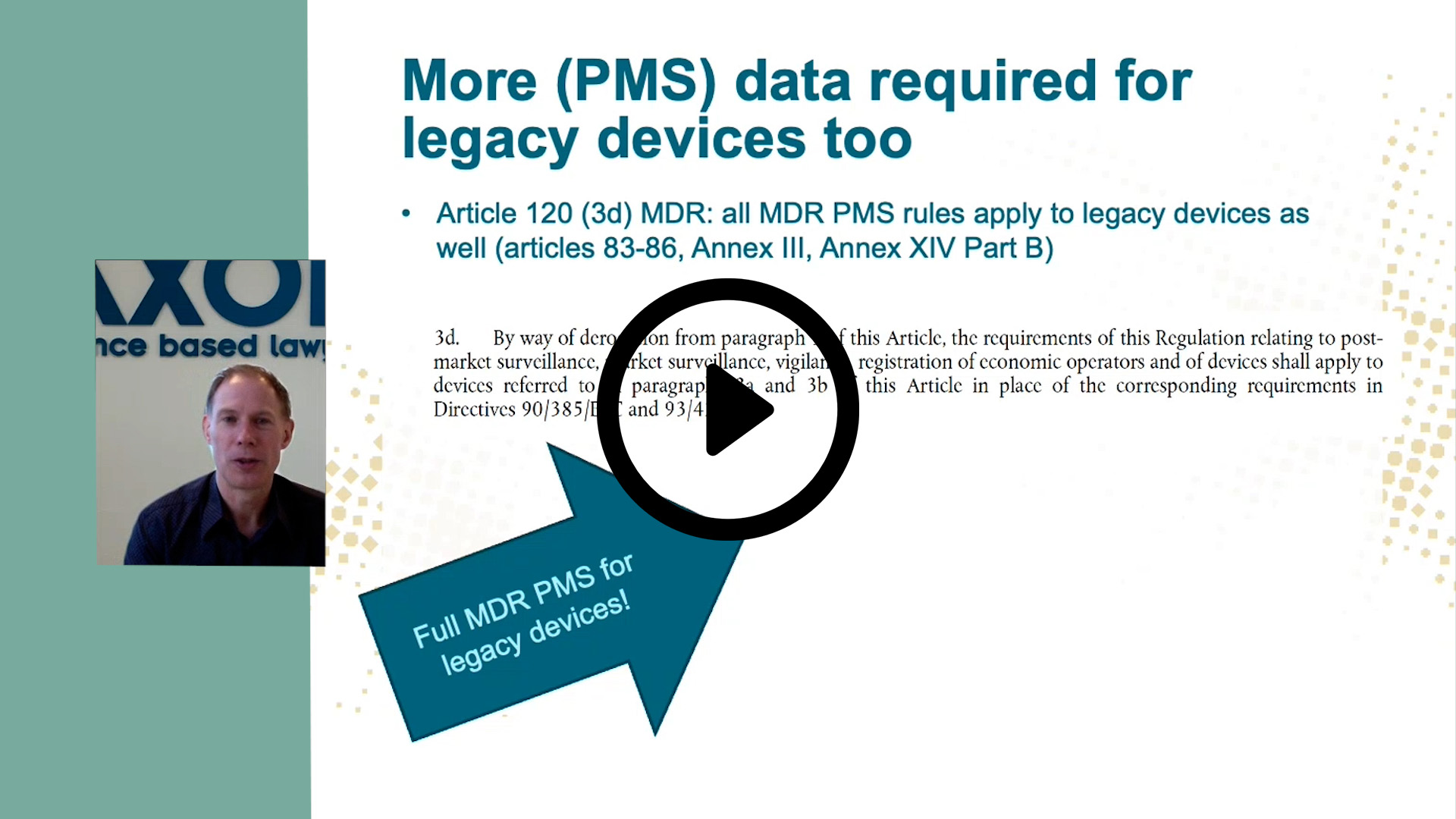
On February 16, EU Parliament voted to extend the transitional period for legacy devices under Article 120 of the EUMDR, giving companies more time to transition to MDR and notified bodies more time to process these applications.
This extension however does not impact certain obligations for manufacturers of marketed MDD legacy devices. Per Art. 120 (3) of regulation (EU) 2017/745 (MDR), post-market monitoring safety and performance according to MDR standards is currently already required for marketed MDD legacy devices, in fact already since 26 May 2021, which not every manufacturer is aware of.
In this webinar, jointly organised by Axon Lawyers and Cascador Health, we’ll cover how to comply with to Post-Market Surveillance (PMS) and Post-Market Clinical Follow-Up (PMCF) requirements and from what place to source the data required. We’ll go into detail about the life cycle concept for PMS and PMCF data required under the MDR, reporting requirements and data sourcing strategies…
We’ll also cover why and how Real World Data (RWD) has a crucial place in the PMS plan and how to access RWD in a GDPR compliant way.
Speakers:
- Erik Vollebregt, Partner at Axon Lawyers
- Laurent Laffineur, Business Development Director, Cascador Health
We look forward to meeting you online!